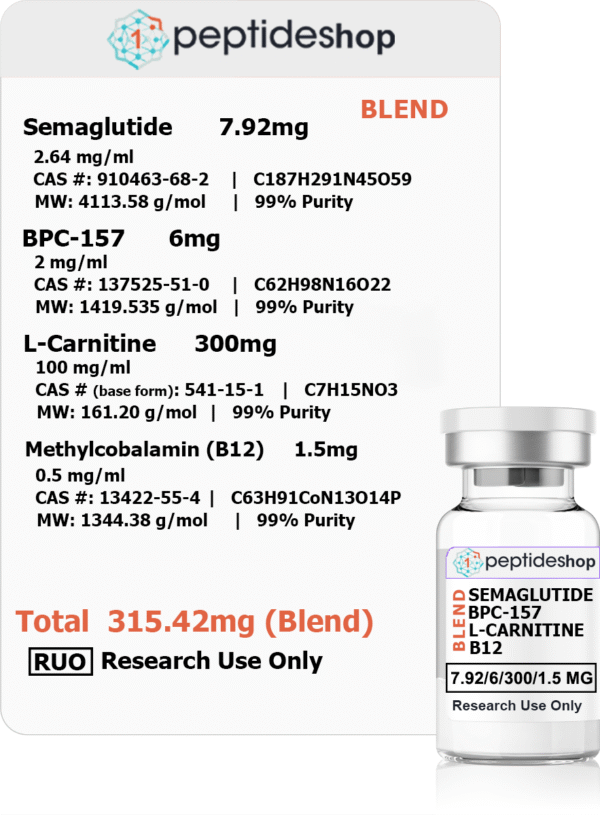
Semaglutide, BPC-157, L-Carnitine, Methylcobalamin (B12) (315.42mg) (Blend)
Original price was: $190.00.$132.00Current price is: $132.00.
This unique formulation combines four clinically significant compounds with complementary mechanisms to support metabolic optimization, cellular recovery, and systemic energy regulation
Contents of the Package
- 1 sterile multi-dose vial
- Total volume: 3 ml
- Formulation (per ml):
- Semaglutide: 2.64 mg
- BPC-157: 2 mg
- L-Carnitine: 100 mg
- Methylcobalamin (Vitamin B12): 0.5 mg
- Diluted in bacteriostatic saline for enhanced stability and multi-dose use
- Packaged with tamper-evident seal and professional labeling
About Semaglutide, BPC-157, L-Carnitine, Methylcobalamin (B12)
This unique formulation combines four clinically significant compounds with complementary mechanisms to support metabolic optimization, cellular recovery, and systemic energy regulation.
- Semaglutide, a GLP-1 receptor agonist, is clinically utilized to enhance satiety, improve insulin sensitivity, and promote sustained weight loss.
- BPC-157, a pentadecapeptide derived from gastric proteins, is associated with accelerated wound healing, GI repair, tendon and ligament regeneration, and anti-inflammatory modulation.
- L-Carnitine, a naturally occurring amino acid derivative, plays a critical role in mitochondrial fatty acid transport, promoting energy production and metabolic flexibility.
- Methylcobalamin (Vitamin B12) supports methylation cycles, red blood cell synthesis, nerve function, and energy conversion at the cellular level.
This combination is designed to synergistically target core systems including metabolic regulation, mitochondrial function, vascular healing, and neuroprotection.
Adverse Reactions
Adverse reactions are generally mild to moderate and often resolve with dose adjustment. Known and potential side effects by component:
Semaglutide:
- Nausea, early satiety, dyspepsia
- Constipation or diarrhea
- Rare: pancreatitis, gallbladder dysfunction
- Less common: fatigue, dizziness
BPC-157:
- Extremely well-tolerated
- Transient headaches, dizziness, or localized injection sensitivity reported anecdotally
L-Carnitine:
- Gastrointestinal discomfort (nausea, diarrhea)
- Rare: fishy body odor due to trimethylamine accumulation
Methylcobalamin (B12):
- Rare hypersensitivity reactions
- Possible acneiform rash or itching at high doses
Mechanism of Action
Semaglutide acts as an analog of human GLP-1 (glucagon-like peptide-1), binding to GLP-1 receptors in the pancreas and brain. It enhances glucose-dependent insulin secretion, inhibits glucagon release, and delays gastric emptying, ultimately reducing appetite and caloric intake. It also activates hypothalamic satiety centers [1][2].
BPC-157 activates angiogenic growth factors, particularly VEGF and FAK-paxillin pathways, supporting capillary regeneration, anti-inflammatory cytokine modulation, and tissue-specific repair. It upregulates eNOS and influences dopaminergic and serotonergic systems [3][4].
L-Carnitine facilitates the transport of long-chain fatty acids into the mitochondrial matrix, essential for beta-oxidation and ATP synthesis. It may also reduce oxidative stress markers and improve insulin sensitivity in skeletal muscle [5].
Methylcobalamin serves as a cofactor for methionine synthase, supporting DNA methylation, myelin sheath maintenance, and homocysteine clearance. It also plays a role in energy metabolism via succinyl-CoA production [6][7].
Benefits
Potential Benefits (Clinically Observed or Theoretically Supported):
- Sustained weight loss via appetite suppression and glucose regulation (Semaglutide)
- Enhanced GI integrity, wound healing, and tendon repair (BPC-157)
- Improved fatty acid metabolism and physical endurance (L-Carnitine)
- Neuroprotection, increased energy, improved mood (Methylcobalamin)
- Antioxidant and anti-inflammatory effects across multiple systems
Side Effects
- GI disturbances, especially during initiation (Semaglutide)
- Rare allergic or sensitivity reactions (B12, L-Carnitine)
- Injection site redness or irritation in some individuals
- L-Carnitine at high doses may result in mild body odor changes
Contraindications & Precautions
This formulation should not be used in individuals with:
- History of medullary thyroid carcinoma or multiple endocrine neoplasia type 2 (due to Semaglutide)
- Severe gastrointestinal disorders (e.g., gastroparesis)
- Known hypersensitivity to any component (e.g., cobalamin allergy)
- Chronic renal failure without medical supervision (for L-Carnitine metabolism)
Caution is advised in patients with pancreatic disorders, or those taking medications that alter gastrointestinal motility.
Drug Interactions
- Semaglutide may slow gastric emptying, potentially delaying the absorption of oral medications (e.g., acetaminophen, antibiotics).
- BPC-157 has shown no known pharmacokinetic interactions to date.
- L-Carnitine may interact with anticoagulants such as warfarin (enhanced effect) [8].
- Vitamin B12 absorption may be impaired by proton pump inhibitors, metformin, or colchicine.
⚠️Co-administration with medications affecting glycemic control should be monitored closely.
Pregnancy & Breastfeeding
There are limited data on the use of Semaglutide or BPC-157 in pregnancy. Animal studies suggest Semaglutide may cause embryofetal toxicity at high doses [9]. BPC-157’s effects on reproductive tissues have not been fully elucidated. Use of this formulation is not recommended during pregnancy or lactation unless under specific clinical justification.
Children
Safety and efficacy of this multi-agent formulation in pediatric populations have not been established. Due to the complexity of hormonal, metabolic, and neurological development in children, this product is not advised for use in individuals under 18 years of age unless under specialized medical supervision.
FDA approval
- Semaglutide is FDA-approved under the trade names Ozempic®, Wegovy®, and Rybelsus® for type 2 diabetes and chronic weight management.
- BPC-157 is not currently FDA-approved but has been extensively studied in preclinical models for regenerative purposes.
- L-Carnitine (Rx version Carnitor®) and Methylcobalamin are FDA-approved as individual compounds for deficiencies and metabolic support.
⚠️This specific formulation is not an FDA-approved combination product and should be used in accordance with professional guidance and applicable regulations.
References
- Nauck MA, et al. (2016). "Glucagon-like peptide-1 (GLP-1) analogs for type 2 diabetes." Endocrine Reviews.
- Davies MJ, et al. (2021). "Once-weekly semaglutide in adults with overweight or obesity." New England Journal of Medicine.
- Sikiric P, et al. (2018). "BPC-157 and healing: Growth hormone, nitric oxide, angiogenesis." Current Pharmaceutical Design.
- Brcic L, et al. (2020). "BPC-157 and its role in neural repair and inflammation modulation." Biomedicine & Pharmacotherapy.
- Mingorance C, et al. (2011). "L-Carnitine supplementation and mitochondrial function." Journal of Physiology and Biochemistry.
- O'Leary F, et al. (2010). "Vitamin B12 in health and disease." Nutrients.
- Scalabrino G. (2009). "Neurobiology of methylcobalamin." Neuroscience Research.
- Harper CM, et al. (2007). "Warfarin interaction with carnitine supplements." Pharmacotherapy.
- FDA Ozempic® Label – Reproductive Toxicology Section. Drugs@FDA Database